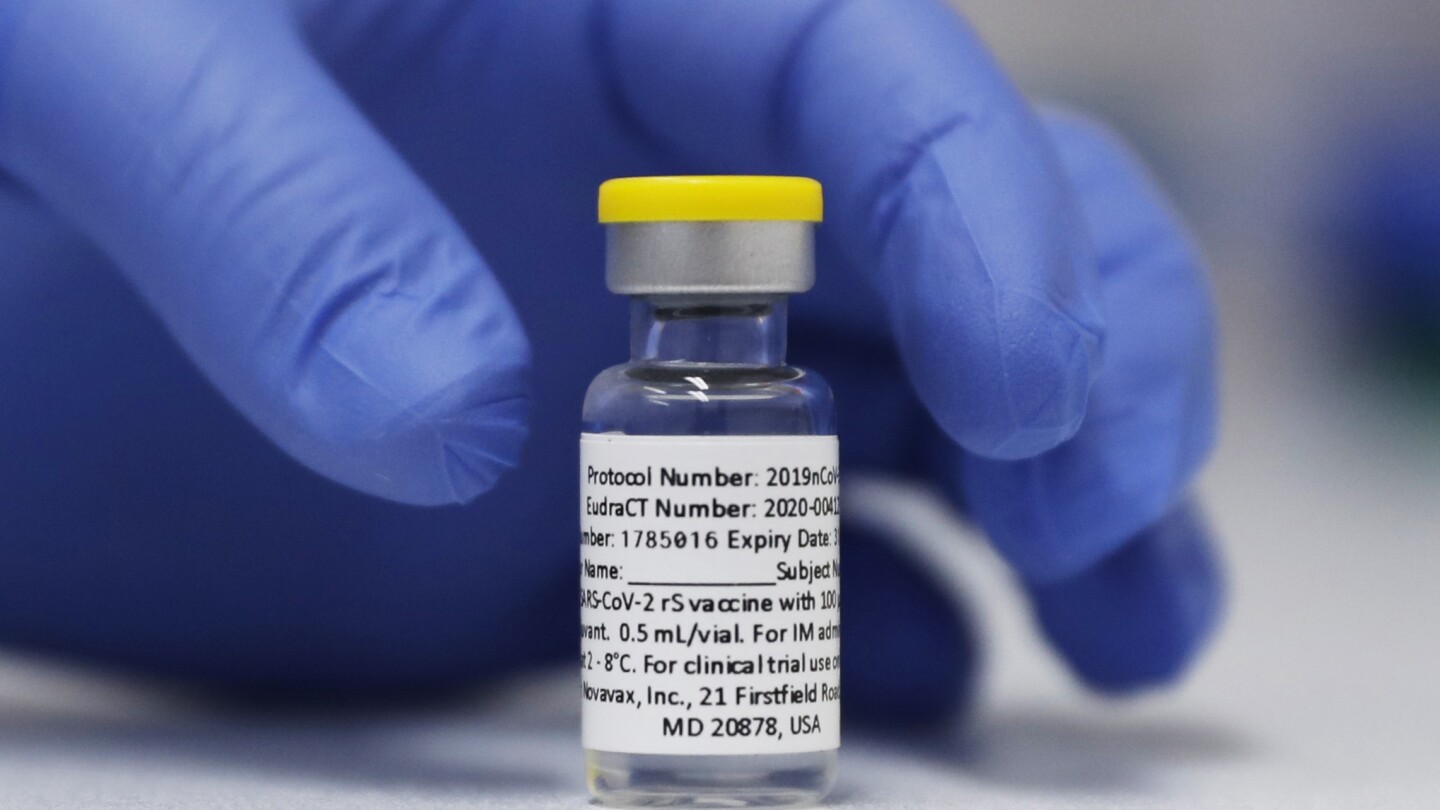
Novavax announced that its protein-based COVID-19 vaccine is progressing toward full FDA approval following additional discussions with the agency. Shares surged over 21% in morning trading after the company stated it is actively working with the FDA to address requests for additional clinical data collection. While Novavax’s vaccine currently operates under emergency use authorization, full approval would allow it to remain on the market indefinitely. The FDA had initially targeted an April 1 approval date but paused the decision, reportedly due to internal discussions. Unlike mRNA vaccines from Pfizer and Moderna, Novavax uses lab-grown spike proteins packaged into nanoparticles, a method familiar in vaccines for hepatitis B and shingles. — new from AP News