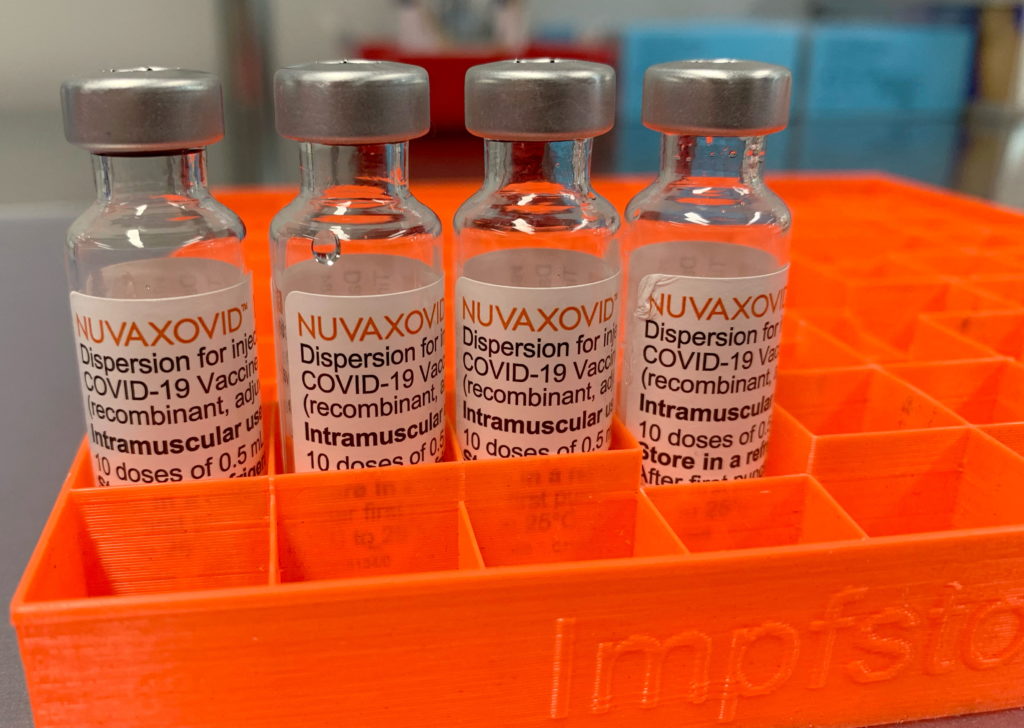
The U.S. Food and Drug Administration (FDA) has granted full approval to Novavax’s COVID-19 vaccine but with specific restrictions. The vaccine is now approved for adults aged 65 and older, as well as individuals aged 12 to 64 with underlying health conditions that increase their risk from the virus. This contrasts with competitors Pfizer and Moderna, whose vaccines are fully licensed for broader age groups. The FDA’s decision reflects concerns over vaccine safety and efficacy, particularly regarding potential links to certain heart conditions. Additional trials are required to further assess the vaccine’s benefits and risks in younger populations without pre-existing conditions.
— new from PBS