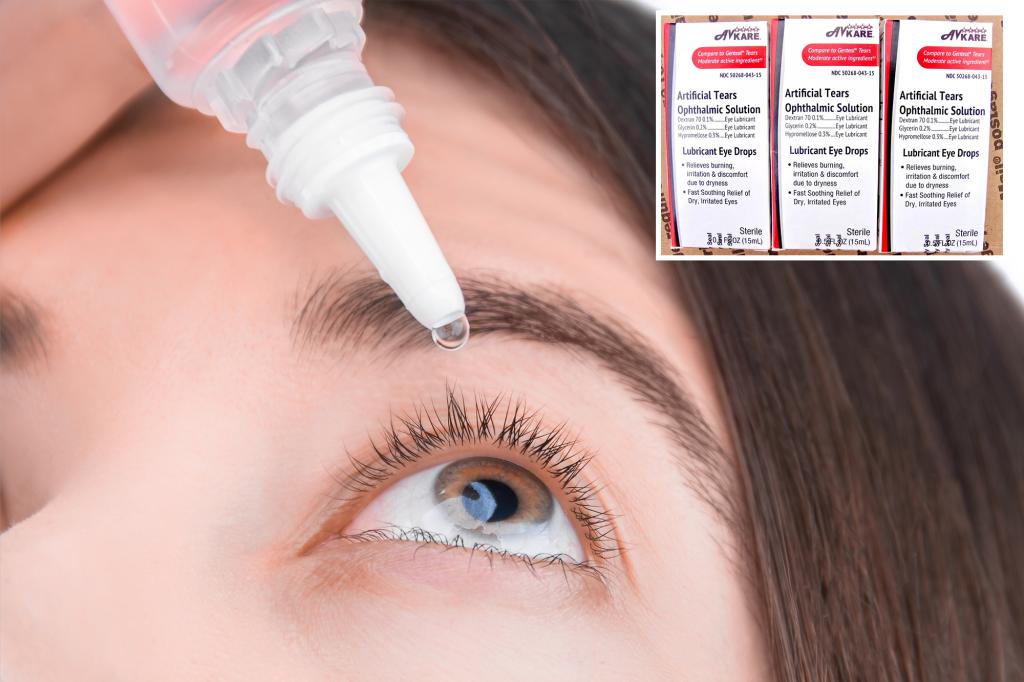
Approximately 76,000 cases of eye care products have been recalled across the United States by BRS Analytical Services following an FDA audit that identified deviations from standard manufacturing practices. The recall includes various eye drops used for relieving itchy and dry eyes due to concerns over sterility assurance. While the immediate health risks are unclear, potential hazards cannot be ruled out. Affected products include Artificial Tears Ophthalmic Solution, Carboxymethylcellulose Sodium Ophthalmic Gel, and others. These products were distributed between May 2023 and April 2025, with expiration dates extending to March 2027. Customers are advised to stop using the recalled items immediately and can return them to AvKare for a full refund, including shipping costs, by completing a form on AvKare’s website.
— new from New York Post